benedict's test positive color|Benedict's reagent : Tagatay Benedict's reagent - Wikipedia "A Tale of Two Cities" is a historical novel by Charles Dickens, set in London and Paris before and during the French Revolution. The novel explores themes of love, sacrifice, and the influence of the past on the present. It is a tale of social and political turmoil, as well as personal redemption and sacrifice. Brief Synopsis Plot Overview
benedict's test positive color,Benedict’s Test- Principle, Procedure, Steps, Results, UsesBenedict's reagent - WikipediaBenedict’s Test- Principle, Preparation, Procedure, and Result
Benedict’s Test- Principle, Procedure, Steps, Results, Uses
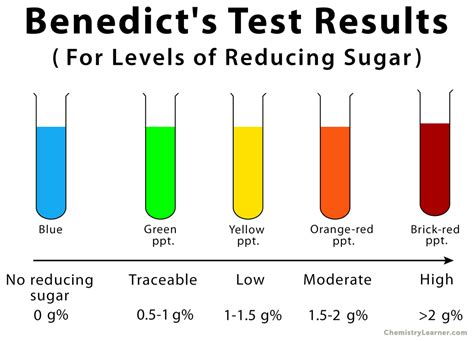
If the color upon boiling is changed into green, then there would be 0.1 to 0.5 percent sugar in solution. If it changes color to yellow, then 0.5 to 1 percent sugar is present. If it changes to orange, then it means that 1 to 1.5 percent sugar is present. If color changes to red,then 1.5 to 2.0 percent sugar is . Tingnan ang higit pa
When Benedict’s solution and simple carbohydrates are heated, the solution changes to orange red/ brick red. This reaction is caused by the reducing property of . Tingnan ang higit paBenedict’s solution is a deep-blue alkaline solution used to test for the presence of the aldehyde functional group, – CHO. One litre of Benedict’s solution can be prepared from 100 g of anhydrous sodium carbonate, 173 g of sodium citrate and 17.3 g . Tingnan ang higit pa Any change in color from blue to green or yellow or orange or red within 3 minutes indicates a positive Benedict test i.e. presence of reducing sugar in the .Benedict's reagent (often called Benedict's qualitative solution or Benedict's solution) is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reag.When exposed to reducing sugars, the reactions undergone by Benedict’s reagent result in the formation of a brick-red precipitate, which indicates a positive Benedict’s test. An .
Benedict's reagent Observation and result interpretation: Positive Benedict’s test: color change from blue to brick red precipitate (glucose) Negative Benedict’s test: no change in color (sucrose) and water. Benedict’s test relies on the ability of reducing sugars to reduce cupric ions (Cu²⁺) present in Benedict’s solution, resulting in a color change from blue to green, yellow, orange, or even brick-red, .Benedict's Test. The Benedict's test can verify the presence of reducing carbohydrates: compounds that have hemiacetals in their structures and are therefore in equilibrium .
Quality Checking: Benedict’s solution is blue in color. In order to check purity of Benedict’s solution take 5 ml of Benedict’s solution in test tube and heat it. If is does .Q.1. Do complex carbohydrates give positive results in Benedict’s test? Ans. Complex carbohydrate like starch and cellulose does not give a positive result in Benedict’s test unless they are broken down through . Benedict’s test is a test used to determine the presence of reducing sugar in any substance. Reducing sugar is a simple carbohydrate with a free aldehyde or ketone group and acts as a reducing agent. . Benedict’s test for reducing sugars. Benedict’s reagent is a blue solution that contains copper (II) sulfate ions (CuSO 4 ); in the presence of a reducing sugar copper (I) oxide forms . Copper (I) oxide is not soluble in water, so it forms a precipitate; Method. Add Benedict's reagent (which is blue as it contains copper (II) sulfate ions) to a sample . Benedict's test is a simple chemical test used to detect the presence of reducing sugars like glucose in a solution. The test result is positive if a brick-red precipitate forms, indicating the .
Which color change represents a positive reaction for the presence of sugar using the Benedict's test? A. . Add a known amount of starch to the unknown sample and then run the Benedict's test. D. Incubate the unknown sample with catalase and then test for proteins. E. Put a small amount of the unknown sample onto a fresh potato slice and .

Benedict's Reagent + BoilReducing Sugars = All Monosaccharides, Lactose & Maltose (not Sucrose)Updated Version 2.0https://youtu.be/ARY0cHpGa_8Red colour chan. A positive test with Benedict’s reagent is shown by a color change from clear blue to brick-red with a precipitate. Generally, Benedict’s test detects the presence of aldehydes, alpha-hydroxy-ketones, and hemiacetals, including those that occur in .

Benedict's test is a simple chemical test that can be used to check for the presence of reducing sugars. . The original color of Benedict's reagent is blue. It turns green, yellow, orange or red, depending on the concentration of reducing sugar present. . Benedict's test positive on the left and negative on the right. Reference. byjus .Then carry out Benedict’s test as normal; add Benedict’s reagent to the sample and heat in a water bath that has been boiled – if a colour change occurs (orange-red precipitate), a non-reducing sugar is present; Explanation. The addition of acid will hydrolyse any glycosidic bonds present in any carbohydrate molecules; The color changes that are seen during this test are the same as with Benedict’s solution. Use dilute sugar solutions with this test (0.02 M). Method: Add 1 ml of the solution to be tested to 5 mL of Benedict’s solution to a test tube and mix well. The test tube is heated in a 55°C water bath for 10–20 minutes.
Serial dilutions. Serial dilutions are created by taking a series of dilutions of a stock solution.The concentration decreases by the same quantity between each test tube . They can either be ‘doubling dilutions’ (where the concentration is halved between each test tube) or a desired range (e.g. 0, 2, 4, 6, 8, 10 mmol dm-3); Serial dilutions are completed . Aldehydes give a positive result, and ketones give a negative result for Benedict’s test. The end result of Benedict’s test is a brick-red colored precipitate. Any chemical compound that is a reducing agent can give a positive result for Benedict’s test. Benedict’s solution has a dark blue color.
Explain why these compounds are not used as solvents in the TCIA test. In benedict test why benedict agent does not reduce the starch? Why does water have a higher boiling point compared to ethanol? Although D-fructose is not a reducing sugar, it usually gives a slightly positive result in Benedict's test if heating is prolonged. Explain. Benedict's solution is originally blue. Any change in color indicates presence of a reducing sugar. The intensity in color change is proportional to the concentration of the sugar. If there is .
Study with Quizlet and memorize flashcards containing terms like Benedict's Test, Iodine test, Sudan IV and more. . The positive color is dark blue/purple/black. Sudan IV. Tests for lipids (fats, oils, and waxes). The negative color is light pink. The positive color is dark pink/red. Biuret Test. Tests for protein. The negative color is light .benedict's test positive colorA positive test with Benedict’s reagent is shown by a color change from clear blue to brick-red with a precipitate. Generally, Benedict’s test detects the presence of aldehydes, alpha-hydroxy-ketones, and hemiacetals, including those that occur in certain ketoses.
Testing a urine sample with Benedict's reagent is a simple way of checking for the presence of glucose in people who are suspected of having this disease. It is, however, not a definitive test, as other reducing sugars will produce the same reaction. If urine tests positive, further tests will have to be carried out to confirm the condition.
- Benedict reagent is used to identify monosaccharides and some disaccharides, including glucose and maltose, respectively. - A positive result for the Benedict test occurs anytime the reagent changes from its original blue color. The reaction requires heat to take place.Difference between Barfoed’s Test and Benedict’s Test. Barfoed’s reagent is similar to Benedict’s reagent except that the pH is lower (around 4.5), and the heating time is reduced to two minutes. Benedict’s test would determine if the sample is a reducing sugar, and Barfoed’s test would determine if it is a monosaccharide or .
Figure 6.48: a) Heating the Benedict's solution in a boiling water bath, b) Benedict's test results: left tube is sucrose (negative), right tube is glucose (positive), c) Negative result, d) Positive result. Conjugated aldehydes are unreactive in the Benedict's test, and the author found many non-conjugated aldehydes to also be unreactive.
benedict's test positive color|Benedict's reagent
PH0 · Benedict’s test: Definition, Principle, Uses, and Reagent
PH1 · Benedict’s Test: Principle, Procedure, Uses, and
PH2 · Benedict’s Test : Principle, Reagent Preparation, Procedure and
PH3 · Benedict’s Test
PH4 · Benedict’s Test
PH5 · Benedict's reagent
PH6 · Benedict's Test: Principle, Requirements, Procedure
PH7 · Benedict's Test
PH8 · 6.4D: Individual Tests